Functional Precision Oncology: Filling the Gap Left By Genomics-Based Approaches
Precision oncology (also known as personalized medicine in cancer treatment) is about matching an individual to the optimal therapy for their unique form of cancer. Many people associate personalized medicine with genetic testing. Genomic tumor profiling has advanced our understanding of cancer in recent years and brought about a whole new generation of targeted anticancer therapeutics. However, in cancer care, the definition of precision oncology has evolved beyond tumor genomics.1 It now includes functional testing options that can help bring greater certainty to precision oncology.
Mainstream Precision Oncology
The overarching goal of personalized strategies is finding a drug or drug combination to combat an individual’s cancer effectively. So, if two individuals have the same type of cancer, an oncologist practicing a personalized approach may use different treatments based on the unique mutations or other biological markers present. Typical precision oncology approaches use whole-genome sequencing or whole-exome sequencing of a tumor or liquid biopsy sample (though there are several other non-sequencing methods commonly used) to capture a snapshot of the mutational status of the bulk tumor at a specific point in time.1,2
Benefits of Static Precision Oncology Approaches
The benefit of these precision oncology techniques is that they generate a mutation profile of an individual’s cancer.1 If that mutation profile includes a gene variant for which there is a targeted treatment, it can be a starting point for first-line therapy based on accepted standards of care. However, the mutation profile will also include many other gene variants that may or may not affect the therapeutic response and may not accurately characterize the full scope of a tumor’s heterogeneity. In addition, this type of analysis is static, meaning that it represents a single moment in time. Because cancer is constantly changing and mutating, over time, the mutations once thought to be driving tumor growth or metastasis may become irrelevant.
For example, if genomic profiling identified a HER2-positive breast cancer, there would be a reason to try trastuzumab targeted therapy, approved explicitly for HER2-positive breast cancer.1 There have been several notable successes in identifying mutations that respond to targeted therapies such as imatinib (for Philadelphia chromosome-positive chronic myelogenous leukemia), vemurafenib (for mutated BRAF V600E melanoma), and erlotinib (for lung cancers with EGFR-activating mutations).1,3
.png?width=222&height=167&name=Genetic%20Testing%20V2%20(1).png)
Figure 1. Genomic profiling can be used as a complementary tool for in-life functional precision oncology approaches. While it is not required, it can be helpful in determining which therapies to test in the lab. Once scientists understand what mutations might be driving an individual’s cancer, bioinformatics can help identify potentially effective drugs beyond the standard of care.
Drawbacks of Static Precision Oncology Approaches
Biomarker-based (genomic or otherwise) approaches to precision oncology face two complex realities. Many patients will not have mutations for which there is a targeted drug, and not all patients with a targeted mutation – and sometimes a tiny proportion – receive clinical benefits. This means that many tumors predicted to be responsive to targeted therapies are not responsive.
Ultimately, this difficulty stems from the inability of many genomics-based methods employed in precision oncology to accurately capture the intra-tumoral heterogeneity of many cancers. Genetic sequencing produces a static representation of tumor genomics at a single point in time, but cancer is complex and dynamic, and its genetic makeup changes over time.4 Understanding a person’s tumor genetics can help narrow down treatment options, but it cannot tell which mutations are driving cancer1 or how various treatment options will interact biologically with tumor tissue or its microenvironment.5

Figure 2. Research shows that most individuals with cancer do not directly benefit from treatment that is informed primarily by genetics. In fact, cancer treatment guided by genomic tumor profiling has a 95%+ outcome failure rate, which means less than 5% of individuals with cancer benefit from having actionable cancer driver genes identified and cancer therapies prescribed.1
What Makes Each Person’s Cancer Unique?
Tumors are typically classified into types or subtypes according to their tissue or organ of origin. For example, cancer originating in the bone or muscle is generally called a sarcoma, versus a carcinoma that develops in the skin or the tissue that lines internal organs (called the epithelium).


Figure 3. Sarcoma originates in the bone or muscle. Figure 4. Carcinoma develops in the skin or epithelium.
While cancers are annotated using this general naming scheme, in reality, even cancers that arise in the same tissue can be vastly different; they may have unique genetics, may exhibit different behavior and may respond unpredictably to treatment.
According to the National Cancer Institute, tumor heterogeneity is a term that describes the differences between tumors of the same type in different individuals, the differences between cancer cells within a single tumor, or the differences between a primary (original) tumor and a secondary tumor. These differences may involve the tumor’s genes and proteins. For example, some cancer cells in a tumor may have genetic changes (called mutations) that are not present in other cancer cells in that tumor. Tumor heterogeneity can play an essential role in how cancer is diagnosed and treated and how it responds to treatment.
Tumors from the same tissue type are heterogeneous.6 For example, one person’s sarcoma or carcinoma can be genetically and functionally distinct from another’s, even if they are the same type of cancer (called inter-tumor heterogeneity).
Moreover, as tumor cells divide and grow, they evolve, accumulating mutations that make them unique. Growth and development over time turn tumors into a heterogenous patchwork of genetic modifications (called intra-tumor heterogeneity), with cells that grow later in cancer development accumulating distinct mutations in addition to the mutations that arose early in tumor development.7 And as these mutations amass, new and different functions, such as metastases, can emerge.6,7
Creating a Clear Clinical Picture with Personalized Cancer Treatments
Inter- and intra-tumoral heterogeneity may be partly responsible for the therapeutic failures of the one-size-fits-all, first-line treatments received by those living with cancer. More personalized approaches have materialized to help treat cancer's unique, individual nature and fill the treatment gap left by standard-of-care therapies.
Because human cancers are incredibly heterogeneous, effective treatment requires a personalized approach. There is growing advocacy for a pivot to Functional Precision Medicine (FPM), which involves exposing live tumor cells from affected individuals to various drugs, then evaluating response to guide treatment decisions.
The Expanding Scope of Precision Oncology: Functional Precision Oncology
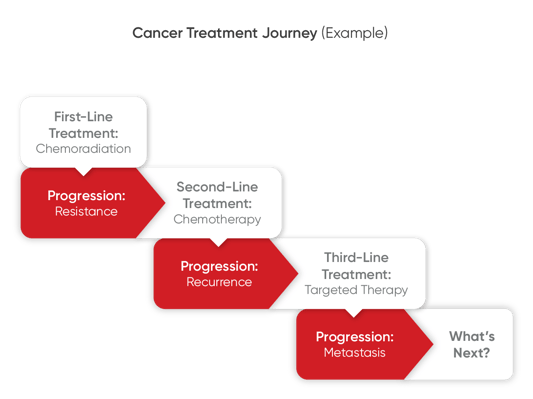
 For many patients who have not benefitted from the standard-of-care treatment, developed drug resistance, or experienced relapses in response to targeted therapies, the obvious question is, what’s next? Functional precision oncology strategies can help answer this question by helping to inform next-line treatment decisions.
For many patients who have not benefitted from the standard-of-care treatment, developed drug resistance, or experienced relapses in response to targeted therapies, the obvious question is, what’s next? Functional precision oncology strategies can help answer this question by helping to inform next-line treatment decisions.
Rather than focusing on a static snapshot of a tumor’s mutational profile, functional precision oncology can capture tumor cell behavior and can be used to directly test selected anti-tumor therapies or combination therapies.1,2 Additionally, functional precision oncology allows researchers and clinicians to couple functional analysis with genomic information and clinical knowledge.1,2,8
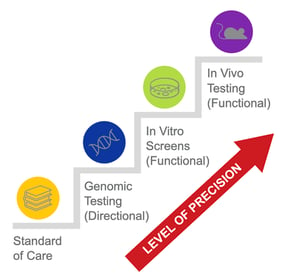
The Different Types of Functional Precision Oncology Models: Benefits and Drawbacks
Thanks to advances in cell culture and other laboratory techniques, there are now multiple functional precision oncology models that take advantage of 2D and 3D culture or transplantation methods using animal models. The precision oncology toolbox includes functional precision oncology methods that are broadly categorized as in vitro (cell culture) or in vivo (in-life) models.

In vitro functional precision oncology models involve the growth of tumor samples from patients in a petri dish or test tube as 2D cancer cell lines, 3D spheroids, or 3D patient-derived organoids and testing their responses to anti-cancer drugs.2 In vitro testing is beneficial for rapidly screening many potential therapeutics. However, many therapeutics that show positive results in vitro may not benefit a patient in the clinic because the tumor cells are isolated and tested in an environment that doesn’t accurately mimic conditions in the human body.9
Figure 5. Cancer cells are bathed in a drug for a predefined period in either a glass vial or petri dish, which is not physiologically equivalent to that of a human body.
 In contrast, in vivo functional precision oncology models use cancer cell lines or patient-derived tumor samples implanted in zebrafish, flies, mice, and other living animals.2 These animals can be then be dosed with anti-cancer drugs. The benefits of an in vivo functional precision oncology approach are that they can accurately imitate the environment from which a tumor grows (or metastasizes) in a patient’s body. Thus, it creates a clinically relevant model to evaluate anti-cancer therapeutics.
In contrast, in vivo functional precision oncology models use cancer cell lines or patient-derived tumor samples implanted in zebrafish, flies, mice, and other living animals.2 These animals can be then be dosed with anti-cancer drugs. The benefits of an in vivo functional precision oncology approach are that they can accurately imitate the environment from which a tumor grows (or metastasizes) in a patient’s body. Thus, it creates a clinically relevant model to evaluate anti-cancer therapeutics.
Figure 6. Patient-derived xenograft (PDX) models are made by implanting a sample of an individual’s tumor into special, immunodeficient mice, typically under the skin (known as subcutaneous transplantation) or in the corresponding anatomic location from which the patient’s tumor originated (known as orthotopic transplantation or the correct place).
Patient-Derived Tumors in the Functional Precision Oncology Landscape
In vivo models have been shown to capture a tumor's inter- and intra-tumoral heterogeneity and associated growth or therapeutic responses more accurately than less advanced models.2 In particular, PDX models have emerged as one of the more accurate in vivo functional precision oncology models.2,10 Large, multi-institutional studies have shown that PDX models, when combined with sequencing methods, can deliver reliable information that translates directly into the clinic.10
Typical Engraftment Rates for PDX Models
If an individual’s tumor tissue stabilizes and sufficiently expands in tissue volume in an animal model, that means engraftment was successful. Engraftment rates (the percentage of time engraftments grow on an animal after three passages) for creating PDX mice vary by cancer type, a common critique of using this technique more broadly.2 In osteosarcomas, for instance, transplantation rates range from 20 to 100%.2,11 For head and neck cancers, engraftment may range from 11 to 100%, depending on whether primary or metastatic tumors were used.12 A study from an Australian group using PDX, coupled with sequencing, to guide treatment decisions in a high-risk pediatric cancer patient population reported a 57% successful engraftment rate.13
![]() Figure 7. Engraftment success can also be affected by tumor sample size.
Figure 7. Engraftment success can also be affected by tumor sample size.
Biopsy samples are usually very small in volume, but have a high viable cell content derived from the chemotherapy-naive tumor. In contrast, tumor tissue samples obtained from surgical resections are more abundant but can show massive necrosis if it is obtained after chemotherapy and must be inspected by an experienced pathologist to select viable tissue.11 In contrast, post-therapy samples may have a higher content of resistant and more aggressive cells, thus resulting in higher rates of PDX establishment.11
PDX in Action: Addressing Rare, Recurrent, Refractory, and Metastatic Cancers in the Clinic
Personalized in vivo testing is generally a good fit for individuals diagnosed with solid-tumor-types of cancer determined by their oncologist to be regional, distant, or have metastatic potential — typically late stage II through stage IV, and who are well enough to undergo a biopsy. For people with rare, recurrent, drug-resistant, and metastatic cancers, functional precision oncology offers options beyond trial-and-error approaches to treatment.
Standard-of-care therapies may not be available for individuals with these types of cancers, let alone targeted therapies. Rare cancers make up nearly a quarter of all cancers and cancer deaths. Still, because there are many fewer of them relative to all cancer patients, they are harder to study, and there is less opportunity to test potential therapies.14 Personalized disease models, like PDX mice, can bridge that gap for rare cancers.
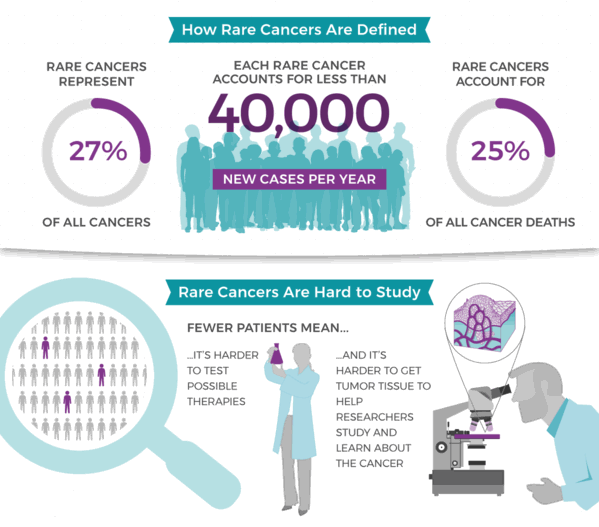
Figure 8. MyPART (My Pediatric and Adult Rare Tumor network) was created to engage individuals with rare cancer in research and unite them with researchers and clinicians commited to the development of effective rare therapies. All pediatric and adolescent cancers are rare, and MyPART focuses on cancers affecting individuals ages 0-39. Source: National Cancer Institute (NCI). cancer.gov/mypart.
PDX models offer the additional benefit of modeling cancer recurrence, resistance, and metastasis. Accordingly, ongoing clinical trials implement PDX models and sequencing methods to investigate their utility in different cancer types, including biliary tract cancer, metastatic prostate cancer, high-grade osteosarcoma, and others. Clinical studies, such as Match-R, have successfully developed PDX models for 33% of enrolled patients with acquired resistance to targeted therapies. Over 90% of those developed models accurately replicate the clinic's disease progression and treatment responses.
YourPDX™: Harnessing the Power of Functional Precision Oncology
Certis Oncology Solutions offers YourPDX testing, a viable functional precision oncology approach for a wide range of solid cancer types. At Certis, we use personalized orthotopic PDX models of an individual’s cancer for testing. Orthotopic PDX models are different from other types of PDX models, such as subcutaneous models, where tumor tissue is engrafted under the skin.

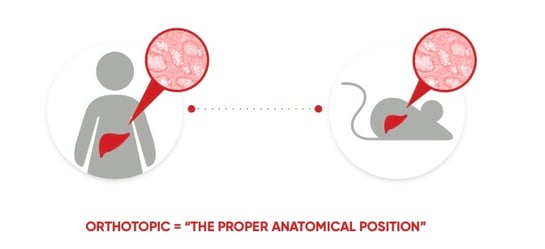
Figure 9. Orthotopic means occurring in the usual place within the body. In orthotopic PDX mouse models, human tumor tissue is engrafted in the corresponding anatomic location in the mouse from which the individual’s tumor originated (in this example, the liver).
Orthotopic patient-derived xenograft (O-PDX) models are considered the gold standard for cancer research and preclinical drug development.15 In a recent letter published in Cancer Cell, cancer researchers from Harvard, MIT and other prominent institutions posited that the local tumor microenvironment (TME) matters in preclinical basic and translational studies. Clinically relevant animal models, such as orthotopic tumor models where tumors develop and grow in their native TME, are more likely to provide reproducible information to guide clinical development and reduce the number of clinical failures and their associated financial and human costs.16
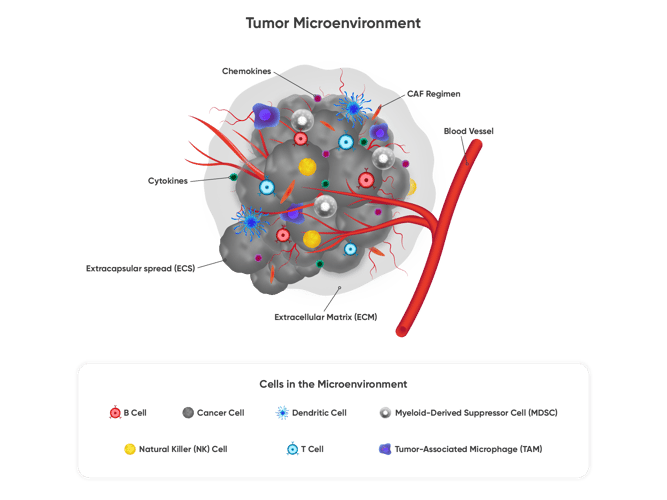 Figure 10. Functional testing using orthoptic PDX mouse models can capture the complexities of tumor tissue and biological interactions that take place in the tumor microenvironment (TME), which is pictured here. The TME is the ecosystem that surrounds a tumor inside the body, which includes the immune cells, the extracellular matrix, blood vessels and other cells, like fibroblasts.
Figure 10. Functional testing using orthoptic PDX mouse models can capture the complexities of tumor tissue and biological interactions that take place in the tumor microenvironment (TME), which is pictured here. The TME is the ecosystem that surrounds a tumor inside the body, which includes the immune cells, the extracellular matrix, blood vessels and other cells, like fibroblasts.
The Certis YourPDX™ Functional Precision Oncology Testing Process
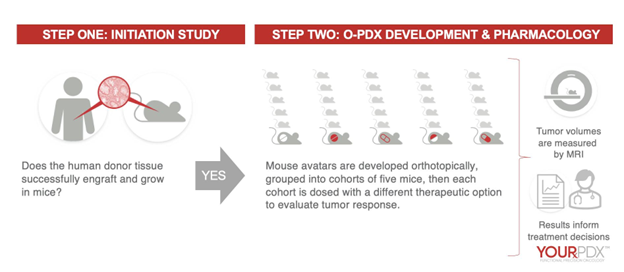
Figure 11. Certis uses its unique surgical and imaging expertise to implant a tumor sample in the mouse tissue corresponding to the human tissue of origin. Certis performs implantation within 24 hours of biopsy or surgery to provide the highest likelihood of success. These mouse avatars can be evaluated for tumor progression, treatment responses against potential treatments, metastasis, and more.
What’s Next FOR YOU OR A LOVED ONE with Cancer? Be Armed with Answers.
YourPDX™ Functional Precision Oncology can help inform next-line treatment decisions. Contact the Certis Clinical Team today to find out more.
 Schedule Meeting
Schedule Meeting  Email: ClinicalServices@certisoncology.com
Email: ClinicalServices@certisoncology.com  Call: 858-228-4110
Call: 858-228-4110
YOURPDX TESTING REQUIRES FRESH TUMOR TISSUE. IT IS ESSENTIAL TO CONTACT US PRIOR TO BIOPSY OR RESECTION SURGERY. YOURPDX IS A FUNCTIONAL TEST THAT MEASURES THERAPEUTIC RESPONSE IN PERSONALIZED PDX MODELS. TEST RESULTS ARE NOT A REPLACEMENT FOR MEDICAL ADVICE. TREATMENT DECISIONS ARE THE RESPONSIBILITY OF PATIENTS AND THEIR PHYSICIANS.
References
1 Letai A, Bhola P, Welm A. Functional Precision Oncology: Testing Tumors with Drugs to Identify Vulnerabilities and Novel Combinations. Cancer Cell. 2022;49(1):26-35.
2 Napoli G, Figg WD, Chau C. Functional Drug Screening in the Era of Precision Medicine. Front. Med. 2022;9:912641.
3 Targeted Molecular Therapy. Penn Medicine website. Accessed October 15, 2022.
4 Ackerman, Sara L. Promising Precision Medicine: How Patients, Clinicians and Caregivers Work to Realize the Potential of Genomics-Informed Cancer Care. New Genetics and Society. 2022. 41:3, 196-215.
5 Mayo Clinic, Genetic Testing — Overview. Mayo Foundation for Medical Education and Research (MFMER). 1998-2021. Accessed 10/27/22.
6 Fisher R, Pusztai L, Swanton C. Cancer Heterogeneity: Implications for Targeted Therapeutics. Br J Cancer. 2013;108(3):479–485.
7 Allison KH, Sledge GW. Heterogeneity and Cancer. Oncology. 2014;28(9):772-8.
8 Pauli C, Hopkins BD, Prandi D et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017;7(5):462-477.
9 Wang X, Zhang H, Chen X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist 2019;2:141-60.
10 Evrard YA, Srivastava A, Randjelovic J, et al. Systematic Establishment of Robustness and Standards in Patient-Derived Xenograft Experiments and Analysis. Cancer Res. 2020;80(11):2286-2297.
11 Landuzzi L, Manara MC, Lollini PL, Scotlandi K. Patient Derived Xenografts for Genome-Driven Therapy of Osteosarcoma. Cells. 2021;10(2):416.
12 Kang HN, Kim JH, Park AY, et al. Establishment and Characterization of Patient-Derived Xenografts as Paraclinical Models for Head and Neck Cancer. BMC Cancer. 2020;20(1):316.
13 Lau LMS, Mayoh C, Xie J, et al. In Vitro and In Vivo Drug Screens of Tumor Cells Identify Novel Therapies for High-Risk Child Cancer. EMBO Mol Med. 2022;14(4):e14608.
14 About Rare Cancers. National Cancer Institute website. Published February 27, 2019. Accessed October 15, 2022.
15 Jäger W, Xue H, Hayashi T, et al. Patient-Derived Bladder Cancer Xenografts in the Preclinical Development of Novel Targeted Therapies. Oncotarget. 2015;6(25):21522-21532.
16 Ho WW, Pittet MJ, Fukumura D, Jain RK. The Local Microenvironment Matters in Preclinical Basic and Translational Studies of Cancer Immunology and Immunotherapy. Cancer Cell. 2022 Jul 11;40(7):701-702.
ABOUT THE AUTHOR
Elie Diner has a PhD in bioengineering and 12 years of research experience in microbiology, synthetic biology and immunology. During his time at the bench, he developed a passion for effective science communication and eventually transitioned into a career as a professional science and content writer. He's authored 12 peer-reviewed scientific publications and numerous blogs, whitepapers, and eBooks for life science companies.
Back to Feed