Precision Oncology as a Model for Bridging the “Valley of Death”
A persistent challenge in anti-cancer drug development remains translation from animal studies to human clinical trials. A shocking 57% of late-stage oncology trials fail due to inadequate efficacy. These failures amount to billions of dollars in unproductive R&D investment and immeasurable human cost—for trial participants as well as patients hoping for the next generation of cancer therapies.
| Cash flow 'Valley of Death' diagram. The cash flow 'Valley of Death' as a function of development stage (time) with typical funding sources at various stages (adapted from [12]). RAID, Rapid Access to Interventional Development; SBIR, Small Business Innovative Research; RAPID, Rapid Access to Preventive Intervention Development; STTR, Small Business Technology Transfer. |
This widely reviewed “Valley of Death” in drug development is generally
acknowledged within the industry, but for decades little progress has been made to bridge it. The scientific community has identified many contributing factors to this failure: irreproducible basic research, poor hypotheses, statistical errors, dis-incentives to report negative results and the use of ambiguous preclinical models, to name a few.
At Certis, we believe the longstanding chasm between bench and bedside can be bridged, and we look to precision oncology as a model for success. Doing our best for individual patients requires a commitment to several fundamental principles: (1) utilizing the most predictive research models available, (2) removing as much subjectivity as possible from results, (3) considering all knowns and unknowns in study design, (4) regarding “negative” outcomes as wins, and (5) prioritizing open and iterative communication between collaborators.
Let’s break that down.
Preclinical models that better represent cancer’s complex physiology.
Preclinical models are an essential tool for providing scientific justification and translational relevance for new and combination therapies. A 2019 review of preclinical studies conducted for anti-cancer drugs over the prior 10 years found that ~80% employed subcutaneous PDX models.
At Certis, we use orthotopic patient-derived xenograft (O-PDX) models. O-PDX models are developed by engrafting human tumor tissue in the corresponding anatomic location in immunocompromised mice. This helps preserve primary tumor characteristics and recapitulates the tumor microenvironment—two often-cited shortcomings of more commonly used cell line-derived and subcutaneous xenografts. While it’s true that O-PDX models require surgical expertise and advanced imaging, the depth and quality of information derived from translational studies is unparalleled.
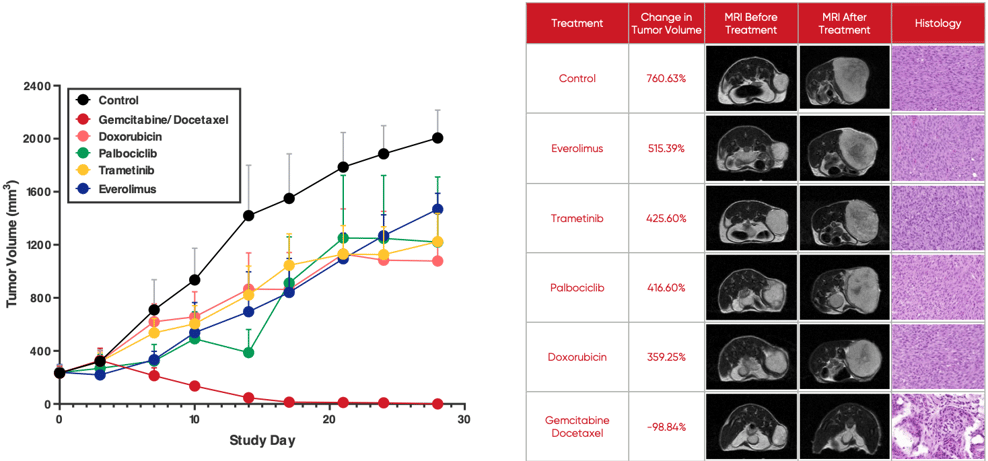
A growing body of evidence shows O-PDX models are not only highly predictive of tumor therapeutic response but can predict drug resistance and tumor recurrence before they become evident in human patients. If leveraged in drug development, this provides an opportunity to inform the development of second-line treatments even as first-line therapies are under development. The image below illustrates how O-PDX models allow for the simultaneous testing of multiple treatment options.
Removing subjectivity from results.
The primary reason given for a preference of subcutaneous over orthotopic PDX models is the ease with which they can be measured. Subcutaneous tumors, implanted in the rear flank of a mouse, are generally measured with calipers. This method is highly variable due to inconsistencies in instrument use: how the animal is restrained, how much pressure is applied with the calipers, and any changes in personnel throughout the study can significantly affect the integrity of the data.
Thanks to modern imaging techniques, measurement of internal tumors in live animals is not only possible, the accuracy of tumor volume measurement has been greatly improved. Using murine-scale MRI technology, we not only obtain consistent, quantitative three-dimensional measurements, but also provide visual proof of tumor response, ensuring confidence in the data produced.
Study designs that account for all knowns and unknowns.
A personalized pharmacology study design takes into account all that is known about a patient: diagnosis, tumor phenotype, treatment history, genomic and biomarker data. It’s how we, in collaboration with the patient’s oncologist, determine how many arms, which drugs to test, route of administration, and dosing strategies. Similarly, a well-designed preclinical efficacy study requires thoughtful consideration of all available data, potential endpoints, and analysis methods to achieve the goals of the primary investigator.
Embracing “negative” results.
Reproducibility (or lack thereof) of published research is a hot topic in biomedical research. This issue has deep roots in the scientific community’s system of incentives and rewards. Publishing basic research is a lever for funding and professional success. But journals have long had a strong bias toward positive results over null hypotheses. Commercial enterprises also have a system of incentives and rewards based upon short-term, personal or departmental success rather than long-term outcomes. In precision oncology, learning which drugs do not elicit tumor response is equally as important as identifying those that do. Even if a study produces no clear therapeutic winner, when we identify drugs that are not effective, we eliminate the suffering and expense that accompanies ineffective treatments. Patients are better off. Appreciating the value of null results and adding that information to our body of knowledge can be critical to clinical success.
Prioritizing open and iterative communication between collaborators.
For decades, the activities of basic and clinical researchers evolved in silos, while pharmaceutical companies focused on building core competencies in regulatory strategy and marketing—and realized most commercial gain. This dynamic contributed to the predominantly transactional, arms-length arrangements that characterized early outsourcing relationships between pharma and service providers. But the nature of these relationships is rapidly evolving for the better, with alliances and public/private collaborations gaining momentum. A critical challenge facing industry is to establish work processes that support open, frequent and iterative communication between collaborators while preserving the necessary level of privacy and protection. Some of this will be achieved through systems and software, but the intangibles that lead to trusting interpersonal relationships are a function of organizational culture and leadership, and this must be prioritized in collaborative relationships.
Bridging the Chasm Between Bench and Bedside
Precision oncology is in many ways a microcosm of anti-cancer drug development. Its well-defined scope and small scale have enabled us not only to apply research to real-life clinical practice, but to test the productive power of collaboration, trust, and the pursuit of scientific truth. At Certis, we believe it stands as testament that the chasm between bench and bedside is solvable.
Learn more about Certis Oncology's services for Drug Development and Translational Oncology.
Back to Feed

![Cash flow 'Valley of Death' diagram. The cash flow 'Valley of Death' as a function of development stage (time) with typical funding sources at various stages (adapted from [12]). RAID, Rapid Access to Interventional Development; SBIR, Small Business Innovative Research; RAPID, Rapid Access to Preventive Intervention Development; STTR, Small Business Technology Transfer.](https://www.researchgate.net/profile/Spack-Edward/publication/26299789/figure/fig3/AS:202712118501376@1425341743143/Cash-flow-Valley-of-Death-diagram-The-cash-flow-Valley-of-Death-as-a-function-of.png)